12.5.2025 / 5-minute read
Implementing a GMP-Compliant, High-Capacity Conveyor System for a Global Pharmaceutical Manufacturer
Overview
Client Profile: A leading multinational pharmaceutical company specializing in high-potency solid dosage forms and biologics, requiring an upgrade to its central logistics hub.
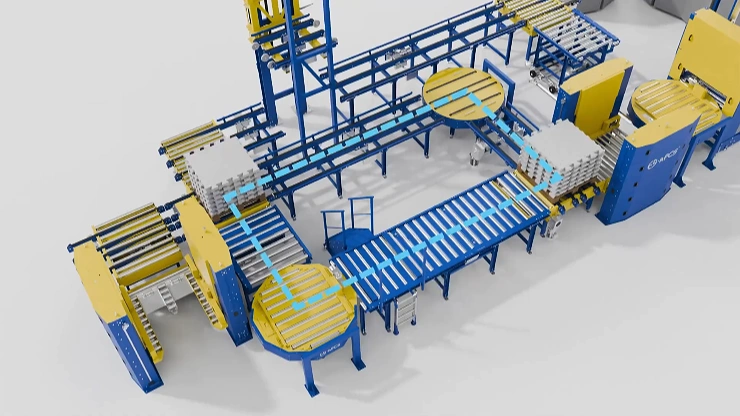
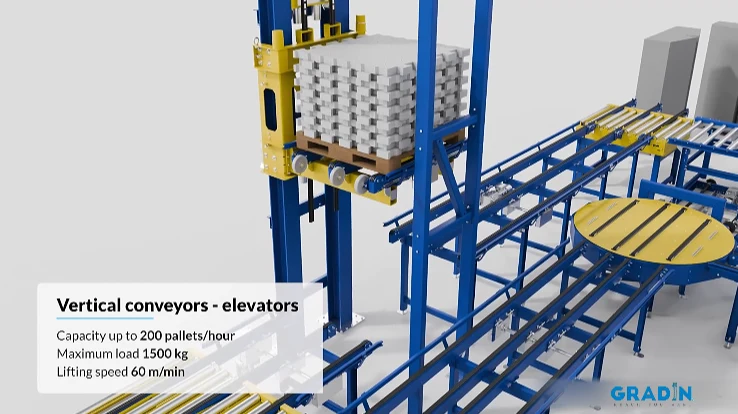
This case study demonstrates how a proven industrial-grade pallet conveyor system—with 180° turntables, high-speed vertical lifts (60 m/min), and a 1500kg capacity—was successfully transformed into a GMP-compliant pharmaceutical logistics solution.
Through strategic modifications including full stainless steel construction, contained vertical transfers, and integrated RFID traceability, it now ensures batch integrity, prevents cross-contamination, and enables reliable, high-throughput (200+ pallets/hour) automated flow for critical pharmaceutical production and distribution environments.
Basic Parameters
| Category | Parameter | Specification |
|---|---|---|
| System Performance | Design Throughput | Up to 200 pallets/hour |
| Maximum Unit Load | 1,500 kg | |
| Vertical Lift Speed | 60 m/min | |
| Key Components | Directional Change | 180° Roller Turntable, 180° Chain Turntable |
| Horizontal Transport | Roller Conveyor, Chain Conveyor | |
| Vertical Transport | Vertical Conveyor / Lift | |
| Pallet Compatibility | Supported Pallet Types | EPAL, EURO, CHEP, Plastic EPAL, Steel Boxes, Box Pallets, and other common industrial pallets. |
| Design Note | Industry Adaptation | A robust platform suitable for heavy-duty logistics, easily adapted for GMP-compliant applications with material and design modifications (e.g., stainless steel construction, containment features). |
Challenge
- Regulatory Compliance: The system had to meet GMP (Good Manufacturing Practice) standards for cleanability, material integrity, and full audit trails.
- Heavy Load Handling: Required reliable transport of full IBC totes (Intermediate Bulk Containers) and palletized raw materials, with individual loads often exceeding 1000 kg.
- Batch Traceability: Mandatory 100% track-and-trace for every pallet (batch) throughout the transfer process.
- Cross-Contamination Risk: Needed to physically and environmentally separate different product zones within the facility.
- Throughput Bottleneck: Manual transfers and slow vertical movement hindered the goal of 24/7 continuous production support.
Highlights
- GMP-Engineered Conveyors & 180° Turntables:
- All contact surfaces—rollers, chains, frames—were constructed from AISI 304/316L stainless steel with electropolished finishes for easy cleaning and corrosion resistance.
- 180° roller and chain turntables were equipped with sealed, low-particulate drives, enabling efficient, space-saving direction changes in high-traffic zones without manual forklift intervention.
- High-Speed Vertical Lift with Containment:
- The critical vertical conveyor (60 m/min lifting speed) was configured as a closed-system airlock. It features interlocked doors and differential pressure control to maintain segregation between warehouse and clean production floors, preventing airborne cross-contamination.
- Universal Pallet Handling with Precision:
- The system was calibrated to gently and securely handle the client’s mixed fleet, including heavy-duty steel box pallets for APIs and standard EPAL/CHEP pallets for packaging materials, all within the 1500 kg maximum load capacity.
- Integrated Track-and-Trace Technology:
- Each key transfer point (turntables, lift entries/exits) was fitted with RFID/2D barcode scanners. This created an automated digital log of each pallet’s journey, timestamps, and location, fulfilling full batch traceability requirements for regulatory audits.